REACh – Registration
Under REACh the manufacturers and importers are responsible for the registration of their chemicals manufactured or imported in amounts of ≥ 1 t per year and to ensure their safe use. This means, that manufacturers and importers must evaluate the risks of their products placed in market and to take measures for the safe handling. For downstream users only safe applications are permitted. Downstream users are responsible to ensure that their intended use is included in the registration.
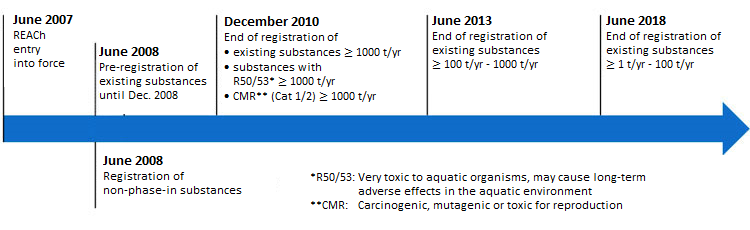
In the first phase in 2008, the pre-registration of phase-in substances ("existing substances" that were commercially available in the EU from 1 January 1971 to 18 September 1981) took place. The pre-registration of substances is important to use the time extensions given for different tonnage bands of the registration. Thus, the production and marketing of the materials used can be carried out until the end of the extended registration deadline. A production and EU-import ban applies to non-(pre-)registered materials. For newly manufactured or imported substances a late pre-registration can be conducted if necessary.
The extended registration period is dependent on the annual tonnage and the dangers posed by the chemicals:
For the registration under REACh, a technical dossier and - for substances with a volume of ≥ 10 tonnes per year - a chemical safety report to the European Chemicals Agency (ECHA) must be submitted. Without registration further use or marketing is excluded.
Our consultation and support services
- Consultation to elements of the REACh regulation
- Dismay analysis
- Communication within the SIEF and with syndicates
- Creation of the registration dossier with IUCLID 6
- Literature research and procurement of relevant data
- Consultation and application of intelligent strategies
- QSAR (Qualitative and quantitative Structure analysis)
- Data waiving
- Read-Across materials
- Assistance with the lab research for studies to be carried out
- Authorising of necessary studies
- Creation of the material security report (for materials > 10 t/yr)
- Exposition consideration and risk characterisation for man and environment (for dangerous materials > 10 t/yr)
- Late pre-registration
- Submission of the dossier with the ECHA
- Communication with the authorities (ECHA, BAuA)